PENICILLIN G BENZATHINE
PRODUCT IDENTIFICATION
41372-02-5 (tetrahydrate)
H.S. CODE
TOXICITY
SMILES
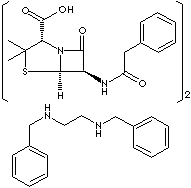
CC1([C@@H](N2[C@H](S1)[C@@H](C2=O)NC(=O)Cc3ccccc3)C (=O)O)C. CC1([C@@H] (N2[C@H](S1)[C@@H](C2=O)NC(=O)Cc3ccccc3)C (=O)O)C.c1 ccc(cc1)CNCCNCc2ccccc2
CLASSIFICATION
Antibiotic, Antibacterial
EXTRA NOTES
Other RN: 2792-46-3, 5968-77-4, 8048-79-1, 12705-12-3, 77087-98-0, 141676-23-5
PHYSICAL AND CHEMICAL PROPERTIES
REFRACTIVE INDEX
GENERAL DESCRIPTION & APPLICATIONS
USA.gov - Penicillin G benzathine
Wikipedia Linking - Penicillin
Google Scholar Search - Penicillin G benzathine
U.S. National Library of Medicine - Penicillin G benzathine
PubChem Compound Summary - Penicillin G benzathine
Drug Bank - Penicillin G benzathine
KEGG (Kyoto Encyclopedia of Genes and Genomes) - Penicillin G benzathine
ChEBI (http://www.ebi.ac.uk/chebi/) - Penicillin G benzathine
NCBI (http://www.ncbi.nlm.nih.gov/) - Penicillin G benzathine
EPA - Substance Registry Services - Penicillin G benzathine
Local:
· Penicillin: Any of a large group of broad-spectrum antibiotic drugs derived directly or indirectly from molds of the genus Penicillium and other soil-inhabiting fungi grown on special culture media, which exert a bacteriocidal as well as a bacteriostatic effect on susceptible bacteria during their growth stage by the inhibition of biosynthesis of their cell wall mucopeptide. Penicillin, beta-lactam antibiotics, possess a four-ring beta-lactam structure shares a nitrogen and a carbon atom with fused a five-membered thiazolidine ring. These antibiotics have low toxicity for the host but effective against most gram-positive bacteria including pathogens
(streptococci, staphylococci, pneumococci); clostridia; some gram-negative gonococci; some spirochetes (Treponema pallidum and T. pertenue); and some fungi. Certain strains of some target species, e.g., staphylococci, secrete the enzyme penicillinase, which inactivates penicillin and confers resistance to the antibiotic.
· Penicillic acid [CAS RN: 90-65-3]: an antibiotic substance produced by several species of Penicillium and Aspergillus; a white solid soluble in water; melting point 83 - 84 C; antibiotic and mycotoxin induceing DNA single-strand breaks, toxic to animal tissues also, causing nephrotoxicity and other damage.
· Penicillin G [also called benzylpenicillin, CAS RN: 61-33-6]: the first and the most widely used penicillin compound for medicinal use. It is used in the form of its stable salts (benzathine, potassium, procaine, and sodium) to treat principally the infections due to penicillin-susceptible gram-positive bacteria, gram-negative cocci, Treponema pallidum, and Actinomyces israelii.
· Penicillin G Benzathine [CAS RN: 41372-02-5]: the benzathine salt of penicillin G; having a long-sustained action, administered orally, intramuscularly. Chemically designation is (2S,5R,6R)- 3,3-Dimethyl-7-oxo-6-(2-phenylacetamido)-4-thia-1-azabicyclo [3.2.0] heptane-2-carboxylic acid with N,N' -Dibenzylethylenediamine (2:1). It is a white crystalline powder; slightly soluble in water and sparingly soluble in alcohol.
· Penicillin G Potassium [CAS RN: 113-98-4]: the potassium salt of penicillin G; administered orally and by intravenously. It is a white crystalline powder; odorless, moderately hygroscopic; soluble in water.
· Penicillin G Procaine [CAS RN: 6130-64-9]: the procaine salt of penicillin G; having a long-sustained action, administered intramuscularly. Chemically designation is (2S,5R,6R)- 3,3-Dimethyl-7-oxo-6-(2-phenylacetamido)-4-thia-1-azabicyclo [3.2.0] heptane-2-carboxylic acid with 2-(Diethylamino)ethyl p-aminobenzoate (1:1). It is a white crystalline powder; slightly soluble in water.
· Penicillin G sodium [CAS RN: 69-57-8]: the sodium salt of penicillin G having a potency of 1500–1750 U per mg; administered intramuscularly and intravenously.
· Penicillin N (also called adicillin, CAS RN: 525-94-0]: a cephalosporin that is more active against gram-negative organisms than penicillin G and is highly active against Neisseria; has been used in the treatment of typhoid fever and gonorrhea.
· Penicillin O: similar to penicillin G in antibiotic action but produced by adding a precursor to the culture medium; penicillin O and its potassium and sodium salts are hypoallergenic.
· Penicillin V: [CAS RN: 87-08-1] a semisynthetic penicillin prepared from cultures of the mold Penicillium in the presence of 2-phenoxyethanol with an autolysate of yeast as the source of nitrogen; a white, crystalline powder, soluble in alcohol and acetone; resists destruction by high humidity (gastric juice), thus orally effective.
· Penicillin V Benzathine [CAS RN: 63690-57-3] : the benzathine salt of penicillin V, administered orally. Chemical designation is [2S-(2a,5a,6b)]-3,3 -dimethyl-7-oxo-6-[(phenoxyacetyl)amino]-4- thia-1- azabicyclo[3.2.0] heptane-2-carboxylic acid with N,N' -Dibenzylethylenediamine (2:1).
· Penicillin V Potassium [CAS RN: 132-98-9]: the potassium salt of penicillin V, administered orally. Chemical designation is [2S-(2a,5a,6b)]-3,3 -dimethyl-7-oxo-6-[(phenoxyacetyl)amino]-4- thia-1- azabicyclo[3.2.0] heptane-2-carboxylic acid, monopotassium salt.
APPEARANCE
IDENTIFICATION
pass
ASSAY (HPLC)
96.0 - 102.0% (61.5 ~ 71.5% + 24.0 ~ 27.0%)
WATER (K.F)
5.0 - 8.0% max
HAZARD OVERVIEW
GHS (Globally Harmonised System) Classification: Acute toxicity, (Oral). Respiratory sensitization. Skin sensitization. Hazard statements: Harmful if swallowed. May cause an allergic skin reaction. May cause allergy or asthma symptoms or breathing difficulties if inhaled..
GHS
Danger
PICTOGRAMS


HAZARD STATEMENTS
H302-H317-H334
P STATEMENTS
P261-P280-P342+ P311
![]()
RISK PHRASES
22-42/43
SAFETY PHRASES
22-26-36/37-45